
A stimulus that causes - or could potentially cause - tissue damage usually elicits a sensation of pain. Receptors for such stimuli are known as nociceptors. Yοu can read more on the structure and function of the somatosensory nervous system here (go on slides 13).
Nociceptors (being free nerve endings) respond to intense mechanical deformation, extremes of temperature, and many chemicals; such as hydrogen cations, neuropeptide transmitters, bradykinin, histamine, cytokines, and prostaglandins, several of which are released by damaged cells or the immune system cells that are transferred to the site of injury. These substances act as binding to specific ligand-gated ion channels on the nociceptor plasma membrane (more on the mechanism of ligand-gated channels can be found here - slide 27, 58 and on).

Image 1. Convergence of visceral and somatic afferent neurons onto ascenting pathways - referred pain
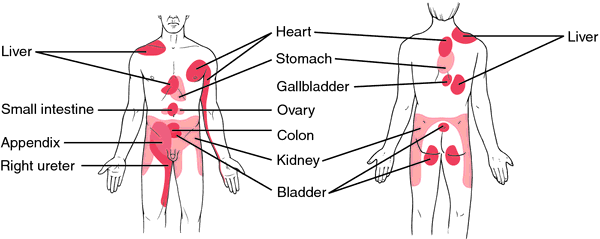
Image 2. Regions of the body surface where we typically perceive referred pain from visceral organs
When incoming nociceptive afferents activate interneurons, it may lead to the phenomenon of referred pain, in which the sensation of pain is experienced at a site other than the injured or diseased tissue. For example, during a heart attack, a person often experiences pain in the left arm, because both visceral afferents from the heart and somatic afferents from the skin (left arm) converge on the same neurons in the spinal cord (Image 1). Excitation of the somatic afferent fibers is the most usual source of the feeling of pain, so we "refer" (we "think") the location of the receptor activation to the somatic source. Image 2 presents the regions of the body surface where we typically perceive referred pain from visceral organs.
Pain differs significantly from the other somatosensory modalities, in the sense that a series of changes occurs in components of the pain pathway that alter the way these components respond to subsequent stimuli - both increased (hyperalgesia) and decreased (hypoalgesia) sensitivity to stimuli can occur. Besides, the pain can last hours and at different intensity after the original stimuli. Moreover, pain can be altered by past experiences, suggestion, emotions, and the simultaneous activation of other modalities.

Image 3. Endogenous opioids
Very interesting is also the fact that specific areas of the CNS can produce a profound reduction of pain by inhibiting pain pathways. In this case, descending pathways that originate in the brain selectively inhibit the transmission of information originating in nociceptors (they inhibit the synaptic transmission between the afferent nociceptor neurons and the secondary ascending inter-neuron). These descending neurons release morphinlike endogenous opioids. Endogenous opioids (like beta-endorphin, dynorphin and enkephalins) are neurotransmitters released by certain neurons in a synapse together with other types. This specific type of neurotransmitters can diffuse away from the synapse and affect other neurons at some distance, in which case they are referred as neuromodulators (Image 3).
This amazing ability of our body to "manage pain" through the endogenous opioids can be seen in the following experiment: when patients were treated with morphine-placebo drug, about 35% of them experienced pain relief. It seems likely that pathways descending from the cortex activate these same regions to exert the placebo effect and inhibiting the transmission of pain at the spinal cord level.
The endogenenous-opioid systems also mediate other phenomena known to relieve pain. In clinical studies, 55-85% of patients experienced pain relief when treated with acupuncture, an ancient Chinese therapy involving the insertion of needles into specific locations on the skin. This technique is thought to activate afferent neurons leading to spinal cord and midbrain centers that release endogenous opioids and other nerotranmitters implicated in pain relief.
Finally, the use of transcutaneous electrical nerve stimulation (TENS) stimulates the non-pain, low threshold afferent nerves leading from a painful site on the surface of the skin leading to the inhibition of neurons in the pain pathways. It is exactly the same phenomenon (but of lower-tech version) when we vigorously rub our scalp at the site of a painful bump on the head.
(Widmaier EP, Raff H, Strang KT. Vander's Human Physiology: the mechanisms of body function. 12 ed.New York: McGraw-Hill International Edition; 2011)
(Widmaier EP, Raff H, Strang KT. Vander's Human Physiology: the mechanisms of body function. 12 ed.New York: McGraw-Hill International Edition; 2011)
No comments:
Post a Comment